FDA Responds to Critical Hemodialysis Bloodline Supply Disruption
The FDA has officially added hemodialysis bloodlines (product code FJK) to the Medical Device Shortage List due to significant supplier disruptions affecting patient access to life-sustaining dialysis treatments. This development highlights the critical vulnerabilities in medical device supply chains and underscores the urgent need for manufacturers to implement robust contingency planning.
What Are Hemodialysis Bloodlines and Why They Matter
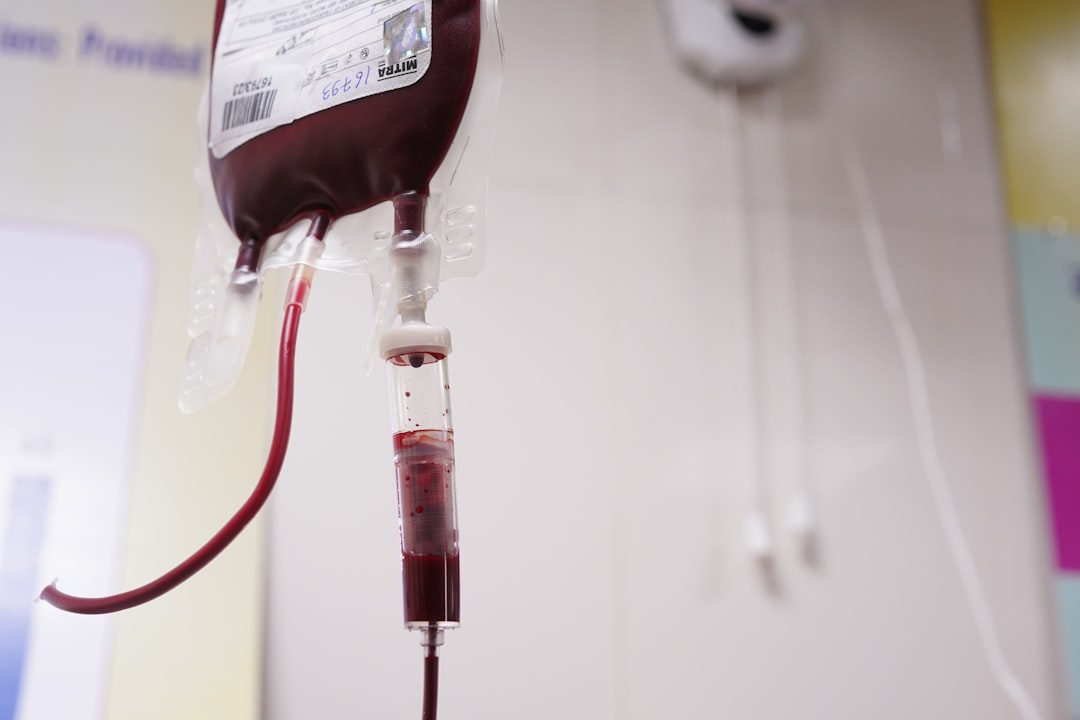
Hemodialysis bloodlines, also known as blood tubing sets with or without anti-regurgitation valves, are essential components that connect patients to dialysis machines during life-sustaining treatments. These Class II medical devices must meet stringent safety and performance standards under FDA regulations, including:
- Biocompatibility requirements per ISO 10993 standards
- Sterility assurance protocols
- Leak-proof connections and pressure resistance specifications
- Clear labeling and traceability requirements
For the approximately 750,000 Americans dependent on dialysis, any disruption in bloodline availability poses immediate health risks and potential treatment delays.
Understanding the Medical Device Shortage List Implications
When the FDA adds a device to the Medical Device Shortage List, it triggers several regulatory mechanisms designed to maintain patient access while ensuring safety:
- Enhanced Supply Chain Monitoring: The FDA increases surveillance of remaining suppliers and manufacturing capacity
- Expedited Review Pathways: Alternative suppliers may receive accelerated 510(k) review for substantially equivalent devices
- Temporary Enforcement Discretion: The agency may exercise flexibility in certain regulatory requirements to prevent patient harm
- Industry Collaboration: Healthcare providers and manufacturers are encouraged to identify alternative products and conservation strategies
Root Causes and Risk Factors
While the FDA has not disclosed specific details about the supplier issues, common causes of medical device shortages include:
- Manufacturing quality issues requiring production shutdowns
- Raw material supply disruptions
- Regulatory compliance violations at manufacturing facilities
- Business decisions to discontinue product lines
- Natural disasters or geopolitical events affecting supply chains
Critical Actions for Medical Device Manufacturers
Immediate Response Steps:
- Assess Your Supply Chain Exposure: If you manufacture hemodialysis equipment or related devices, evaluate your bloodline suppliers and identify potential alternatives
- Review Shortage Contingency Plans: Ensure your quality management system includes procedures for managing supply disruptions per ISO 13485 requirements
- Consider Market Opportunities: Manufacturers with relevant capabilities should assess the potential for entering this critical market segment
Long-term Compliance Strategies:
- Diversify Supplier Networks: Reduce single-source dependencies through qualified alternate suppliers
- Enhance Risk Management: Update your ISO 14971 risk management processes to include supply chain vulnerabilities
- Strengthen Post-Market Surveillance: Monitor market conditions and competitor issues that could impact your product availability
- Engage with FDA Proactively: Maintain open communication channels with the agency regarding potential supply issues
Regulatory Compliance Considerations
Manufacturers must balance the urgency of addressing shortages with maintaining regulatory compliance. Key considerations include:
- Ensuring any alternative products meet equivalent safety and effectiveness standards
- Maintaining proper documentation and change controls during supply transitions
- Coordinating with healthcare providers on product substitutions
- Reporting any adverse events or quality issues promptly to the FDA
The hemodialysis bloodline shortage serves as a stark reminder of the critical importance of supply chain resilience in medical device manufacturing. Proactive planning, diversified sourcing, and robust quality systems are essential for protecting both patients and business continuity in an increasingly complex regulatory environment.


No comments yet. Be the first to comment!